How do Fire Starters Work?
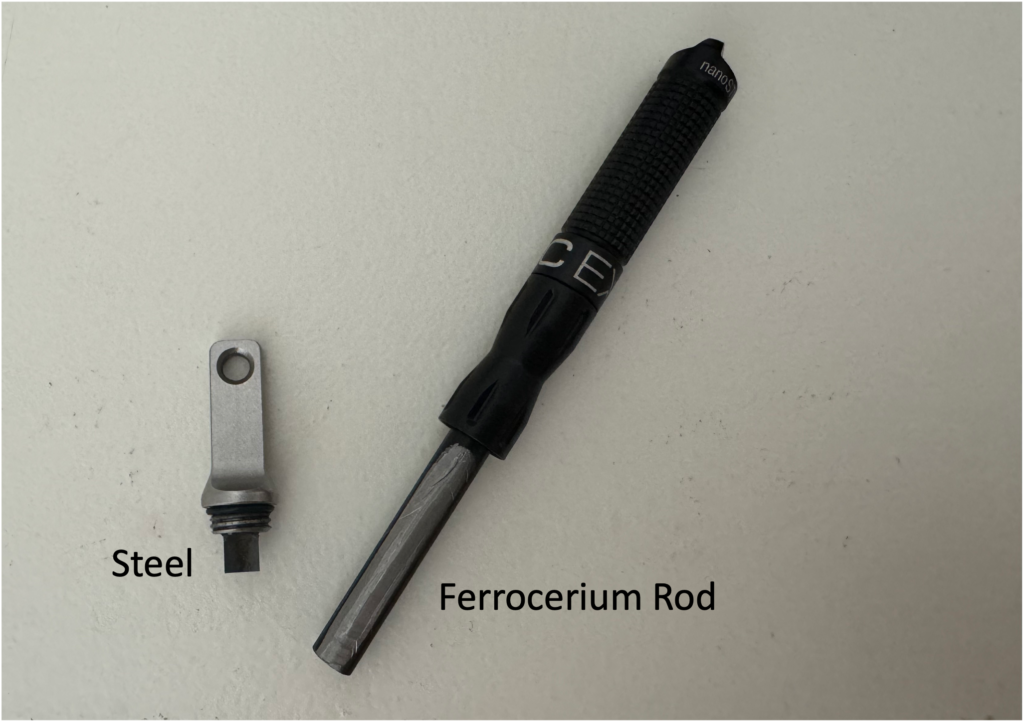
Flint and steel, ferrocerium rods, and magnesium are tools that give you the power to make fire. This article explains the science behind fire starters like the one you see in the photo to the right.

What are Fire Starters?
Fire starters are tools that can be used to start a fire. There are many different types of fire starters including flint and steel, ferrocerium rods, and magnesium fire starters.
Flint and steel describes a tool consisting of a flint rod and steel striker. Flint is a hard rock composed of quartz and steel is an alloy of iron and carbon (an alloy is a metallic substance made of two or more elements that have been melted together).
Ferrocerium rods (also called ferro rods) are an alloy made of lanthanides (cerium, lanthanum, and neodymium) and iron. Ferrocerium rods typically come with a steel striker like the set shown in the image to the right.
Magnesium fire starters contain a bar of magnesium that burns extremely hot once ignited. Magnesium fire starters typically come with an embedded ferrocerium rod in order to spark the magnesium fragments.
How do Fire Starters Work?
The video below shows how a ferrocerium rod works to start a fire. The same principle applies flint and steel, and magnesium fire starters.
How do Ferrocerium Rods Work?
When using a ferrocerium rod, a piece of steel is used to strike the ferrocerium. This contact causes small shards of the ferrocerium rod to be shaved off.
These fragments react with oxygen in the air in a process called oxidation.
Oxidation is a chemical reaction between an element or compound and oxygen. For example, the iron in ferrocerium rods reacts with oxygen to produce iron oxide (more commonly known as rust).
The energy produced in this reaction heats up the shaved off ferrocerium shards and produces hot sparks. If these sparks fall onto a flammable material they can ignite it and start a fire.
How does Flint and Steel work?
When using flint and steel, the steel strikes the flint rod. Since flint is harder than steel, this causes small shards of iron from the steel to be shaved off.
These iron shards oxidize when they react with the oxygen in the air producing iron oxide and heat. The energy produced in this reaction heats up the iron oxide and produces hot sparks that can ignite flammable materials like kindling and start a fire.
The biggest difference between ferrocerium rods and flint and steel fire starters is that ferrocerium rods produce hotter sparks. This is because of the lanthanides like cerium in ferrocerium which have a lower ignition temperature than the iron in steel.
How do Magnesium Fire Starters Work?
Magnesium in its metal form burns very easily in air. This is because magnesium reacts with the oxygen in air to produce magnesium oxide and heat.
However, in order to start this oxidation reaction, the magnesium metal needs a source of energy. This energy comes from the sparks formed by striking a ferrocerium rod (which is typically embedded in one side of the magnesium block).
When using a magnesium fire starter, the user first scrapes off small pieces of magnesium then directs sparks from the ferro rod towards the magnesium in order to ignite it.
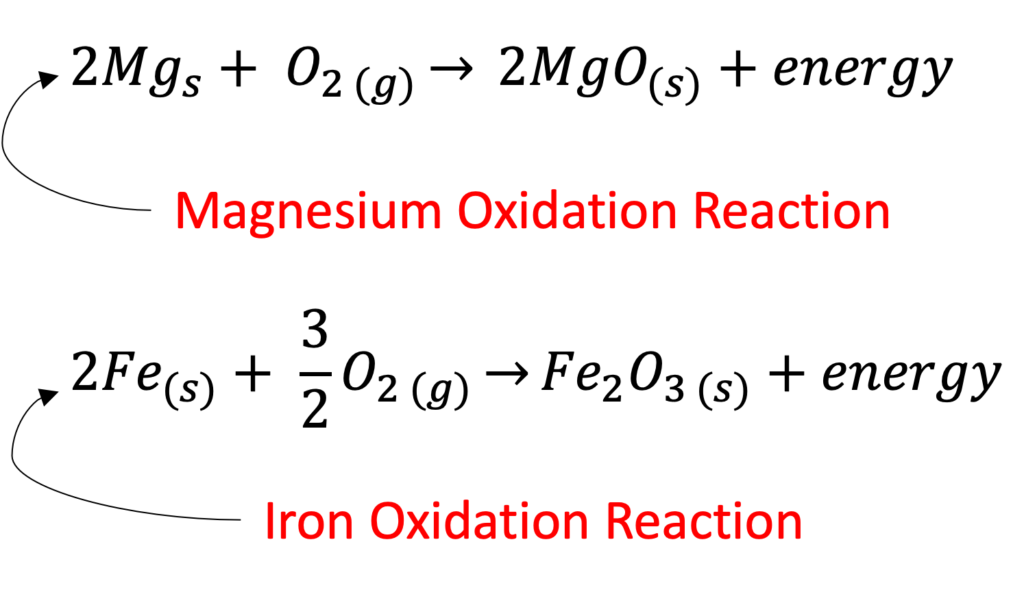
Oxidation Reactions in more detail:
Each of the fire starters explored in this article undergo an oxidation reaction to create heat. Oxidation and reduction reactions (also called redox reactions) are an incredibly important class of chemical reactions.
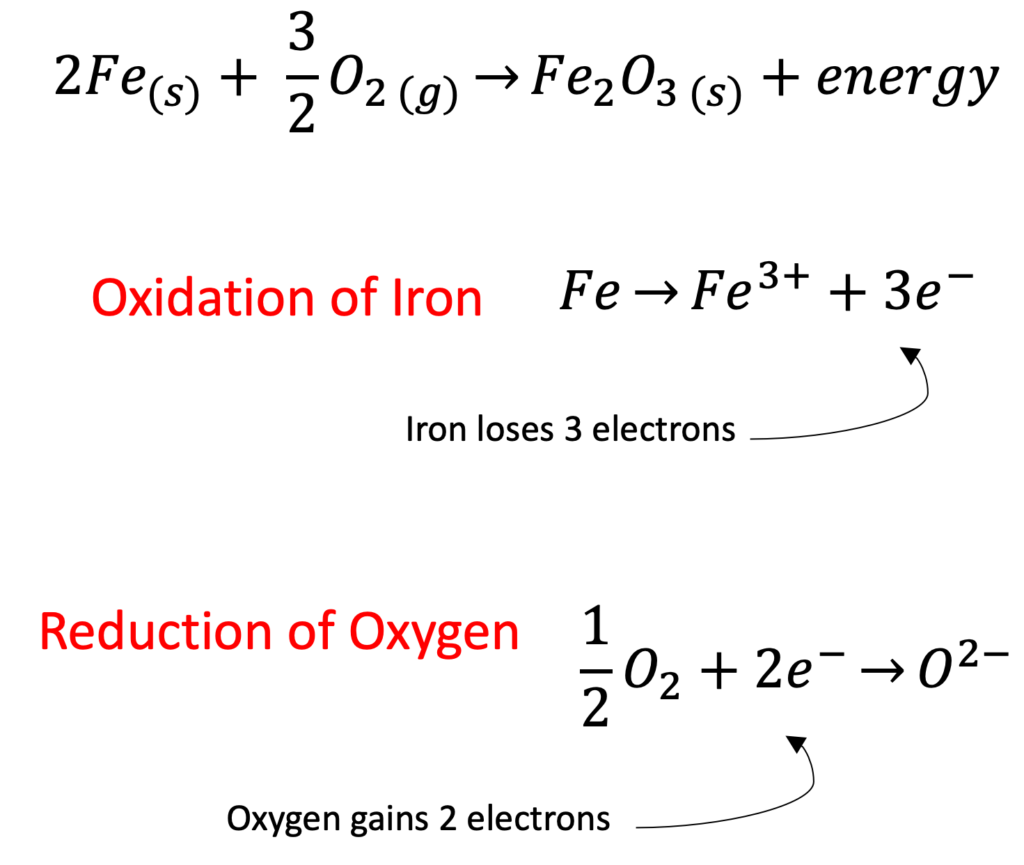
Oxidation occurs when an element or compound loses electrons. Reduction occurs when an element or compound gains electrons.
A redox reaction is one in which one element gains electrons (reduction) and another loses electrons (oxidation).
The oxidation of iron is a great example of a redox reaction because iron is oxidized (each iron atom loses 3 electrons) while oxygen is simultaneously reduced (each oxygen atom gains 2 electrons).
In Summary...
Fire starters are incredibly useful tools that can help keep you warm and well fed on outdoor adventures (or in survival situations). You can buy traditional flint and steel fire starters (or make them yourself), ferrocerium rods, or magnesium fire starters. Ultimately each relies on the chemistry of oxidation reactions to create hot sparks that ignite flammable materials and start a fire.
References:
- Britannica, T. Editors of Encyclopaedia (2014, May 5). chert and flint. Encyclopedia Britannica. https://www.britannica.com/science/chert
- Wente, E. F. , Nutting, . Jack and Wondris, . E.F. (2024, June 24). steel. Encyclopedia Britannica. https://www.britannica.com/technology/steel
- Wikipedia contributors. (2024, September 3). Ferrocerium. In Wikipedia, The Free Encyclopedia. Retrieved 17:15, September 21, 2024, from https://en.wikipedia.org/w/index.php?title=Ferrocerium&oldid=1243805679
- Sean McCoy (2015, April 16). Tutorial: Magnesium Fire Starter. Gear Junkie. https://gearjunkie.com/camping/magnesium-ferrocerium-fire-starter
- BBC Bitesize. (2024). Oxidation. https://www.bbc.co.uk/bitesize/articles/zpbgg7h#zy3gg7h
- Laurel Hamers. (2024, April 27). Why does striking flint against steel start a fire?. Live Science. https://www.livescience.com/chemistry/why-does-striking-flint-against-steel-start-a-fire
- Bushcraft USA. (2010, February 15). Compare flint and steel to ferro rods. https://bushcraftusa.com/forum/threads/compare-flint-and-steel-to-ferro-rods.9338/
- Charles Ophardt. (2024). Burning Magnesium. LibreTexts Chemistry. https://chem.libretexts.org/Ancillary_Materials/Demos_Techniques_and_Experiments/Lecture_Demonstrations/Burning_Magnesium
- Catherine Smith. (2024). Iron: a fiery future | 14–16 years. Royal Society of Chemistry. https://edu.rsc.org/resources/iron-a-fiery-future-14-16-years/4014458.article
- Antagonizer. (2013). Making a Flint and Steel From an Old Carbon Steel File. Autodesk Instructables. https://www.instructables.com/Making-A-Flint-And-Steel-From-An-Old-Carbon-Steel-/
